Asthma
Respiratory disease is a global issue and international networks are critical to informing best-practice approaches to the clinical care and management of childhood respiratory health.
Centre researchers have led the Global Lung Function Initiative (GLI) over the past decade to develop all‐age, multi‐ethnic reference equations for each of the major clinical lung function tests—resulting in the improvement and standardisation of clinical care for children with breathing difficulties world-wide. Locally, we are also working with metropolitan and remote Indigenous communities to establish baseline metrics for lung function and disease prevalence in these at-risk populations.
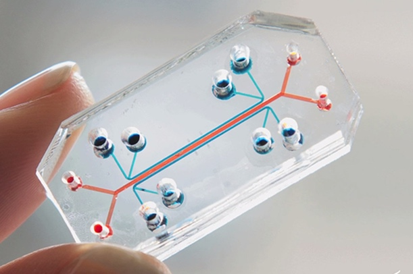
To identify therapeutic strategies to treat childhood asthma, we need to understand the underlying biological mechanisms driving the disease, test new classes of drug that can prevent or repair the damaged airways and advance the most promising candidates into clinical trials. Informed through appropriate clinical cohort studies with biobanking and functional assessments to understand the physiology of the disease, we are implementing cutting-edge ‘lung-on a chip’ technology (a miniaturised model of a living human lung, mounted on a microchip) to investigate the effects of new drugs, tissue repair responses, the absorption of airborne particles, and the inflammatory response triggered by microbial pathogens.
Using such approaches, we have discovered a new class of drug that can boost the intrinsic immunity of asthmatic airways and protect against injury by viruses. Used prophylactically, this can be considered a type of asthma ‘vaccine’. This type of research has the potential to save billions of dollars from the national healthcare budget, improve the lives of millions of children, and make a significant reduction in the Indigenous health gap.
Other teams within the Respiratory Centre are working on alternative approaches to develop an ‘asthma vaccine’. This includes leading the systems biology arm of a $30M USD clinical trial designed to evaluate whether an oral bacterial extract given to high risk infants (n=1,000) for two years can prevent the subsequent development of asthma. And we are about to launch yet another clinical trial (POWER) to determine whether a different bacterial extract (OM-85) can reduce the excessive immune responses to respiratory viruses that triggers acute wheezing in some individuals.
Discover. Prevent. Cure.